A lire sur: http://www.technologyreview.com/view/515381/metals-become-molecular-like-at-the-atomic-scale-reveal-materials-scientists/
One of the defining characteristics of metals is the way they are held together. Essentially, a lattice of metal ions sits in a sea of delocalised electrons and this acts as a kind of glue that binds the structure together.
These “metallic bonds” are entirely different from the covalent bonds that hold molecules together. For a start, metallic bonds are a collective phenomenon that come about because of the bulk behaviour of metal ions and delocalised electrons.
What’s more, metallic bonds have no preferred direction. That’s handy because it makes metal structures stable while also allowing atoms to be easily added or taken away.
By contrast, a covalent bond forms between two atoms and is highly directional. These bonds make up the skeleton that holds molecules together and allows the creation of highly complex structures, such as buckyballs and proteins.
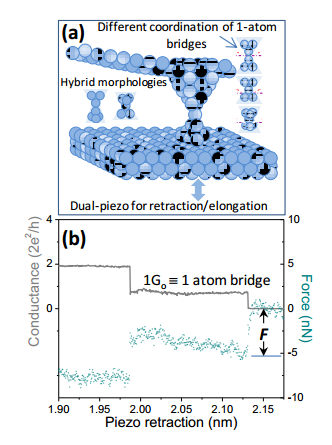
But what of the bond that holds together the simplest metal structure imaginable–two metal atoms forming a bridge? Today, Harsh Deep Chopra and pals at The State University of New York at Buffalo say they have characterised the nature of this bond at room temperature for the first time.
Their astonishing discovery is that the bond between two metal atoms becomes molecular-like in these situations– in other words it is much stronger and highly directional. And they say this finding has important implications for the design and construction of future atomic-sized devices.
Their experiment is straightforward in principle. They brought the tip of an atomic force microscope just close enough to a gold surface that it created a single-atom bridge. They then pulled the tip away to measure how much force it took to break the bridge.
The results are surprising. The force required to break the bonds between gold atoms in a bulk metal is about 0.5 nanonewtons. But when two gold atoms are isolated in a bridge, it takes about 2 nanonewtons to do the same job. That’s or four times the force, which has to be applied in a specific direction. (They got similar results with silver.)
So the bonds between metal atoms in an atomic-scale device are both directional and significantly stronger than those in the bulk material. In other words, the bonds become molecular-like at this scale.
That could have important implications for the way atomic scale devices are designed and built. “The directional bonds provide high configurational stability to atomic-sized metallic devices,” say Chopra and co.
What’s more, these devices are less likely to suffer from the complications arising from bulk metal interfaces which can end up binding with all kinds of unwanted detritus. That simply can’t happen with directional bonds.
Of course, these metal bonds soon lose their directional character when more metal atoms are added. So an interesting question is how far this phenomenon can be exploited to make molecular-like structures. That’s a problem for future work.
But with the trend in miniaturisation for microelectronics now approaching the atomic level, a better understanding of how atomic scale devices are held together will surely not go amiss.
Ref: http://arxiv.org/abs/1305.5582: Metallic Bonds Become Molecular-Like In Atomic-Sized Devices
Atomic force measurements show that the bond between two gold
atoms is highly directional and molecular-like, a finding with
significant implications for the design and construction of atomic-scale
devices

One of the defining characteristics of metals is the way they are held together. Essentially, a lattice of metal ions sits in a sea of delocalised electrons and this acts as a kind of glue that binds the structure together.
These “metallic bonds” are entirely different from the covalent bonds that hold molecules together. For a start, metallic bonds are a collective phenomenon that come about because of the bulk behaviour of metal ions and delocalised electrons.
What’s more, metallic bonds have no preferred direction. That’s handy because it makes metal structures stable while also allowing atoms to be easily added or taken away.
By contrast, a covalent bond forms between two atoms and is highly directional. These bonds make up the skeleton that holds molecules together and allows the creation of highly complex structures, such as buckyballs and proteins.
But what of the bond that holds together the simplest metal structure imaginable–two metal atoms forming a bridge? Today, Harsh Deep Chopra and pals at The State University of New York at Buffalo say they have characterised the nature of this bond at room temperature for the first time.
Their astonishing discovery is that the bond between two metal atoms becomes molecular-like in these situations– in other words it is much stronger and highly directional. And they say this finding has important implications for the design and construction of future atomic-sized devices.
Their experiment is straightforward in principle. They brought the tip of an atomic force microscope just close enough to a gold surface that it created a single-atom bridge. They then pulled the tip away to measure how much force it took to break the bridge.
The results are surprising. The force required to break the bonds between gold atoms in a bulk metal is about 0.5 nanonewtons. But when two gold atoms are isolated in a bridge, it takes about 2 nanonewtons to do the same job. That’s or four times the force, which has to be applied in a specific direction. (They got similar results with silver.)
So the bonds between metal atoms in an atomic-scale device are both directional and significantly stronger than those in the bulk material. In other words, the bonds become molecular-like at this scale.
That could have important implications for the way atomic scale devices are designed and built. “The directional bonds provide high configurational stability to atomic-sized metallic devices,” say Chopra and co.
What’s more, these devices are less likely to suffer from the complications arising from bulk metal interfaces which can end up binding with all kinds of unwanted detritus. That simply can’t happen with directional bonds.
Of course, these metal bonds soon lose their directional character when more metal atoms are added. So an interesting question is how far this phenomenon can be exploited to make molecular-like structures. That’s a problem for future work.
But with the trend in miniaturisation for microelectronics now approaching the atomic level, a better understanding of how atomic scale devices are held together will surely not go amiss.
Ref: http://arxiv.org/abs/1305.5582: Metallic Bonds Become Molecular-Like In Atomic-Sized Devices

Aucun commentaire:
Enregistrer un commentaire